Hygiene Guidelines for the Manufacturing of Non-Sterile Pharmaceutical Preparations in Pharmacies (version of 19 January 2000)
When producing pharmaceutical preparations appropriate measures have to be taken in order to ensure their microbiological quality. Microbial contaminated products can perish and harm the user when applied.
A recommendation for the microbial purity of pharmaceutical products is given in the European Pharmocopoeia in chapter 5.1.4 "Microbiological Quality of Pharmaceutical Preparations" (8, 11). Additional documents are the guidelines for the "Good Manufacturing Practice" of pharmaceutical products in the PIC-GMP-manual (17) and supplementing guidelines (18) for liquids, creams, ointments and other drug preparations as well as the guidelines for the Good Pharmacy Practice in Europe (30). Drug acts, pharmacy operating decrees and comparable legal norms of central European countries (4, 10, 16, 25, 26) demand a hygienically faultless condition of the preparation premises and controlled preparation processes.
All guidelines only emphasize priorities. Model instructions always have orientating character; they have to be adapted and completed according to operational specifications. The application of the stipulations has to be effected directly at the place of work by the working person striving for optimal effectiveness. For that purpose practical experience as to working sequence, knowledge in microbiology and hygiene and a high degree of personal motivation is necessary. The objective of each employee should be to continuously improve safety and effectiveness of the working process.
The guidelines serve for the purpose of information and as a recommendation for the preparation within the areas of prescription and Defektur in pharmacies. For the preparation of sterile drugs and cytostatic agents additional measures are necessary. Likewise the treatment of tea-drugs is to be regulated operationally in a different manner.
The pharmacy head is responsible for instruction and control in the field of hygiene. All employees are obliged to adhere to the hygiene concept and to contribute to the improvement of the hygiene status.
The preparation area is to be kept in a clean and orderly state any time. It has to be in a condition as to space that the risk of microbial contamination is kept as low as possible, preparation premises are to be given preference.
Walls, ceilings, floors and working spaces in the manufacturing area have to be provided with even surfaces and must be easily cleanable. The manufacturing area is to be cleaned in accordance with cleaning and disinfection plans.
Only persons should stay in preparation rooms and areas who perform corresponding activities. No tea-drugs are processed or manufactured, packaged or stored.
In preparation areas only those devices and materials are to be kept which are necessary for manufacturing. As a matter of principle devices are to be exclusively used for the purpose for which they have been designed within the preparation process. A minimal germ formation has to be ensured at parts which have immediate contact to the product by disinfection with alcohol-water mixtures at appropriate concentrations [e.g. 2-propanol 70 per cent (V/V)] or other adequate measures - preferably right before use. A disinfection in advance (e.g. the sterilisation of devices or components of glass, porcelain or metal) is admissible at contamination-safe storing.
Specific cleaning instructions have to be established for machines and devices in particular. This equally applies for scales and water baths in view of their high microbic contamination potential.
It has to be ensured that no persons work within the preparation area who suffer from infectuous diseases or have open skin injuries at uncovered parts of the body. Hands have to be cleaned and disinfected immediately before starting the preparation work and after each work interruption. At the washbasins dispensers with skin protecting washing lotion and disinfectant solution as well as one-way towels have to be available. Hand disinfection is effected as so-called surgical disinfection before working at the open product. The disinfectant is applied after the washing of hands, the thorough rinsing of the washing lotion with warm water and the drying of hands.
Hygiene clothing is to be worn for all preparation processes. It should only be worn in the preparation areas. In addition it is advisable not to enter preparation premises or areas with walking shoes. Special measures of personnel and washing hygiene are to be layed down in working instructions.
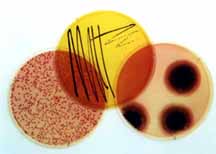
Foto: LQS GmbH, Eschborn
All preparation processes are to be carried out rapidly and if so possible without interruption in areas determined for this purpose. If interruptions cannot be avoided, open products have to be covered in an appropriate manner (watch glass, film). If suitable closed systems for preparation are to be given preference as far as possible. The open product mustn't be touched with bare hands. It is not allowed to sneeze, cough or talk close to the open product. Storing vessels should only remain open for the time necessary for the working process. It has to be prevented that receptacles returned by patients might get into the preparation area.
The necessary hygiene status is to be possibly ensured when purchasing primary materials; as required, measures regarding a germ count reduction have to be taken. Attention is to be paid to the fact that primary materials are not deteriorated relating to their purity status when taking samples or partial quantities.
Exceptional attention is to be focused on the microbial purity of water. A separate operating instruction is to be established for the preparation, safekeeping and storing of water as drug component. This instruction is to take into consideration the demands of the pharmacopoeia and if necessary it justifies reasons for differing regulations.
Exterior packagings (secondary packing materials) of drug and auxiliary substances are to be removed before bringing these substances into the preparation area. In case this is not possible the packaging (primary packing materials) has to be cleaned and disinfected. Reusable storage vessels are to be cleaned and disinfected respectively sterilized before each refilling.
The primary packing material is an essential part of ready-to-use products and mustn't influence the stability of products in an inadmissible way. The delivery receptacle should allow a hygienical product removal and easy application by the user. It has to be chosen in a way that the product quality is ensured also at application and during the application period. For the packaging of semi-solid preparations preferably aluminium tubes the interior of which is protected by a coating or dispenser cans with small openings are to be used. Packing materials are to be purchased in complete sales units the quality of which has been verified by test certificates. Open packing material units have to be stored in a way that a subsequent contamination can be excluded. A reuse of delivery receptacles has to be excluded as a matter of principle. For a reuse by way of exception of for example glass bottles, detailed instructions as to cleaning and sterilisation respectively disinfection outside of the preparation area as well as regarding product storing have to established.

Foto: LQS GmbH, Eschborn
If necessary reusable storage vessels have to be transported outside of the preparation area for cleaning and sterilized or disinfected with alcohol of suited concentration. The alcohol has to be completely evaporated before filling of the product.
Waste is to be collected in special suitable containers. The waste containers are to be emptied daily. In case no waste bags are used, the waste containers are to be cleaned on a daily basis and disinfected at least once a week. The waste containers should not be touched during preparation.
10 Professional continuous training
An internal training in the field of industrial hygiene has to be organized in pharmacies at least once a year. The hygiene concept has to be explained to new employees.
Subject of the training should be:
o Repetition and consolidation of basic knowledge
o Changes by technical developments
o Internal examination results
o Operational working instructions
o Latest findings from spezialized literature
The training attendance is to be confirmed by the participants.
Important working instructions are to be documented operationally, in particular:
o Cleaning and hygiene plan
o Measures concerning the personnel hygiene
o Sterilisation instructions
o Dealing with water for pharmaceutical purposes
It may be useful to incorporate individual hygiene measures into preparation instructions and operation instructions for devices.
Documented should be regularly:
o Cleaning and disinfection measures subject to continuous repetition (type
of measure, date and signum)
o Carrying out of sterilisations (object and conditions of sterilisation, duration,
date, signum)
o Repetition tests for the efficacy of sterilisators
o Maintenance and cleaning processes for water-recycling plants
o Professional continuous training in the field of industrial hygiene
12 Hygiene monitoring and self-inspection
The result of the hygiene measures should be proven by microbiological examination. Self-inspections regarding industrial hygiene are to take place at least once a year by means of a questionnaire.
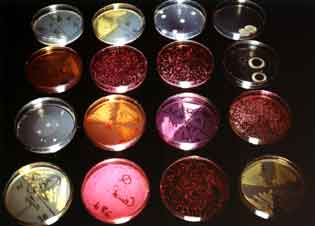
Foto: LQS GmbH, Eschborn
o Glossary and definitions
o Master Hygiene Plan I - Personnel Hygiene
o Master Hygiene Plan II - Cleaning and Disinfection: Devices, Preparation Rooms
and Equipment
o Master Check List for Self-inspection and securing of the operational Hygiene
Concept
o Elaboration of the Guidelines
14 Applicable standards, reference documents and literature
With regularly updated reference documents the latest version has to be considered.
| 1. | Anonym, Richtige Händedesinfektion, PTA heute 7 (1997) 728. |
| 2. | Ausschuss „Arzneimittel-, Apotheken- und Gefahrstoffwesen" der Arbeitsgemeinschaft der Leitenden Medizinalbeamten der Länder, Pharmazeutische Begriffsbestimmungen neu gefasst, Bundesgesundhbl. 35 (1992) 158. |
| 3. | Aye, R.-D., Graeber, B.: Hygienemaßnahmen in der Apotheke, Dtsch. Apoth. Ztg. 130 (1990) 2117-2124. |
| 4. | Bundesgesetz vom 2. März 1983 über die Herstellung und das Inverkehrbringen von Arzneimitteln (Arzneimittelgesetz) der Bundesrepublik Österreich. |
| 5. | Bundesvereinigung Deutscher Apothekerverbände (Hrsg.), Neues Rezeptur-Formularium (NRF), Abschnitt 1.2.7. Hygiene, Loseblattsammlung auf dem Stand der 16. Erg. 1999, Govi-Verlag Pharmazeutischer Verlag, Frankfurt/Main / Deutscher Apotheker-Verlag, Stuttgart. |
| 6. | disinfectant-Kommission der Deutschen Gesellschaft für Hygiene und Mikrobiologie (DGHM), disinfectant-Liste der DGHM. Stand 1.1.1999, mhp Verlag, Howsbaden, 1999. |
| 7. | Felsing, H.-H., Kaitzis, G., Hygienepläne für ambulant-operative Praxen, Dt. Derm. 45 (1997) 827-834. |
| 8. | Europäisches Arzneibuch 1997 (3. Ausgabe), einschl. Nachtrag 1999. Amtliche deutsche Ausgabe, Text 5.1.4., Deutscher Apotheker Verlag, Stuttgart / Govi-Verlag, Eschborn. Anmerkung: Die amtliche deutsche Fassung steht hier stellvertretend auch für die amtlichen Ausgaben des Europäischen Arzneibuches in der Bundesrepubik Österreich und in der Schweiz. |
| 9. | Könemann, A., Sonnenschein, B., Planung und Erstellung eines Hygienekatasters in der pharmazeutischen Industrie, Pharm. Ind. 60 (1998) 795-800. |
| 10. | Gesetz über den Verkehr mit Arzneimitteln (Arzneimittelgesetz - AMG) der Bundesrepublik Deutschland, insbesondere §§ 54 und 55 AMG. |
| 11. | Gay, M., Mikrobiologische Reinheit der Arzneipräparate, Schweiz. Apoth. Ztg. 131 (1993) 581 - 584. |
| 12. | Kommission des Bundesgesundheitsamtes "Erkennung, Verhütung und Bekämpfung von Krankenhausinfektionen" Anforderungen der Hygiene an die funktionelle und bauliche Gestaltung und den Betrieb von krankenhauseigenen und das Krankenhaus versorgenden Apotheken, Bundesgesundhbl. 32 (1989) 30-31. |
| 13. | Kommission Deutscher Arzneimittel-Codex, DAC-Annex H. Qualität von Behältnissen aus Glas, und DAC-Probe 16. Prüfung von Behältnissen auf mikrobielle Verunreinigung, in Bundesvereinigung Deutscher Apothekerverbände (Hrsg.), Deutscher Arzneimittel-Codex (DAC), Loseblattsammlung mit Stand vom 1.11.1999, Govi-Verlag Pharmazeutischer Verlag, Frankfurt/Main / Deutscher Apotheker-Verlag, Stuttgart. |
| 14. | Lingnau, J. (Hrsg,), Hygieneunterweisung im Pharma-Betrieb, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1991. |
| 15. | Robert Koch-Institut - Bundesinstitut für Infektionskrankheiten und nicht übertragbare Krankheiten, Liste der vom Robert Koch-Institut geprüften und anerkannten disinfectant und -verfahren. 13. Ausgabe vom 15. 6. 1997, Bundesgesundhbl. 40 (1997) 344-358. |
| 16. | Pharmacopoea Helvetica, 8. Ausgabe 1997, einschließlich Supplement 1999. Vom Schweizerischen Bundesrat erlassene Deutsche Ausgabe, Eidgenössisches Departement des Innern (Hrsg.), Eidgenössische Drucksachen- und Materialzentrale, Bern. Anmerkung: Auf GMP bei der Arzneimittelherstellung verweist der Ph.-Helv.-Text 1.3. Mit einem eidgenössischen Heilmittelgesetz in der Schweiz wird Anfang 2001 gerechnet. |
| 17. |
PIC, Bekanntmachung des Leitfadens einer Guten Herstellungspraxis für pharmazeutische Produkte der Pharmazeutischen Inspektions-Convention (PIC-GMP-Leitfaden) vom 10.8.1990, BAnz. Nr. 214a (1990). |
| 18. | PIC, Bekanntmachung von ergänzenden Leitlinien zum Leitfaden einer Guten Herstellungspraxis der Pharmazeutischen Inspektions-Convention (PIC). Ergänzende Leitlinien für die Herstellung von Liquida, Cremes und Salben, Pharm. Ztg. 137 (1992) 917. |
| 19. | Rotter, M., Händehygiene, Haut- und Schleimhautdesinfektion, Krankenhauspharmazie 10 (1989) 422-426. |
| 20. | Scheer, R., Mikrobiologische Prüfverfahren im Apothekenmaßstab. Tips für hygienisch einwandfreies Arbeiten, Dtsch. Apoth. Ztg. 127 (1987) 1523-1528. |
| 21. | Schmidt, M., Produktionshygiene - auch in Rezeptur und Defektur, PTA heute 6 (1992) 250-253. |
| 22. | Schöffling, U., Arzneimittelherstellung. How läßt sich der Keimgehalt verringern?, PTA heute 1 (1987) 16-20 u. 100-107. |
| 23. | Schüller, F., Hygiene in der Rezeptur, Dtsch. Apoth. Ztg. 139 (1999) 826-828. |
| 24. | Seyfarth, H., Kritische Anmerkungen zu den Hygieneanforderungen des EG-Leitfadens einer guten Herstellungspraxis für Arzneimittel, Pharm. Techn. Journal Nr. 4/1990 (1990) 10-18. |
| 25. |
Verordnung über den Betrieb von Apotheken (Apothekenbetriebsordnung - ApBetrO) der Bundesrepublik Deutschland, insbesondere § 4 ApBetrO. |
| 26. | Verordnung des Bundesministers für soziale Verwaltung im Einvernehmen mit dem Bundesminister für Land- und Forstwirtschaft vom 4. Juli 1934, betreffend den Betrieb von Apotheken (Apothekenbetriebsordnung) der Republik Österreich, insbesondere § 3 Apothekenbetriebsordnung. |
| 27. | Wallhäußer, K. H., Praxis der Sterilisation, Desinfektion - Konservierung, 5. Auflage, Georg Thieme Verlag, Stuttgart, New York, 1995. |
| 28. | Widmer, H.-R., Grob- und Feindisinfectant - eine aktuelle Übersicht, Pharm. Ztg. 141 (1996) 873-881. |
| 29. | Witte, C., Hygiene und ihre Durchführung in der Apotheke, Dtsch. Apoth. Ztg. 134 (1994) 2725-2728. |
| 30. |
ZAEU - Zusammenschluss der Apotheker in der Europäischen Union, Gute Apothekenpraxis in Europa - Good Pharmacy Practice in Europe (G.P.P.). Abschnitt 2 Richtlinien, 2.2 Allgemeine Anforderungen an die Apothekenbetriebsräume und 2.3 Allgemeine Anforderungen an das pharmacy staff. Nicht offizielle deutsche Übersetzung des ZAEU-Dokumentes seitens der Österreichischen Apothekenkammer. Fundstelle der Originalfassungen in englischer und französischer Sprache: www.pgeu.org, Unterverzeichnis "activities", Stichwort "publications". |
| 31. | Zentrallaboratorium Deutscher Apotheker, ZL-Standards zur Qualitätssicherung bei der Herstellung und Prüfung von Arzneimitteln in der Apotheke. Empfehlungen der Bundesapothekerkammer. Stand: Februar 2000. Publikation in Vorbereitung. |
Annex
Elaboration of the Guidelines
The guidelines have been prepared by the working group "Hygiene" of the Gesellschaft für Dermopharmazie [GD] (Society for Dermopharmacy) — Department "Extemporaneous preparations."
Members of the working group:
• Herr Apotheker Dr. B. Hünerbein, Naumburg, (centralized control),
• Herr Regierungspharmazierat K.-H. Borosch, Schweinfurt,
• Herr Apotheker Dr. H. Döben, Bonn,
• Frau Apothekerin R. Eifler-Bollen, Eschborn,
• Herr Apotheker Dr. H. Reimann, Eschbom,
• Frau Apothekerin Dr. U. Schöffling, Trier,
• Frau Dipl. Biolog. F. Schüller, Bonn,
• Frau Dipl. Biolog. Dr. N. Sievers, Eschborn.
Cooperation and advice:
• Herr Apotheker Dr. K. Albert, Eschborn,
• Herr Regierungspharmazierat J. Lehmann, Augsburg,
• Herr Mag. pharm. Dr. E. Leitner, Hown (Austria),
• Frau Dipl. Biolog. E. Oberkötter†, Eschborn,
• Herr Regierungspharmaziedirektor R. Völler, Darmstadt
• Herr Apotheker Dr. M. Wicki, Egerkingen (Switzerland)
• Herr Apotheker C. Witte, Gründau-Lieblos.
Annex
Glossary and Definitions
| Designation | Definition/abbreviation | Reference | Synonyma/comments |
| Operation procedure | Instruction in writing, describing the realization of certain recurrent activities. | Standard operating procedure (SOP); operation instructions. | |
| Industrial hygiene | Hygiene in pharmacy operations, especially characterized by the hygiene concept. | ||
| Disinfection | Measure for the selective reduction of germ counts with the objective of preventing a transmittance of infectious germs (pathogenic germs) by their deadening (this applies to a virus). | (27) | Sanitation |
| Hand disinfection, surgical | Hand disinfection for the reduction of bacteria flora foreign to skin and characteristical for skin. | Necessary for works at the open product | |
| Hand disinfection, hygienical | Disinfection measure for the deadening of germs after skin contact with infectious material before carrying out cleaning measures. | (27) | e.g. when coming back from the lavatory |
| Preparation | Manufacturing is the producing, preparing, processing, refilling including filling, packaging and marking. | (2) | Preparing; preparation process |
| Preparation area | Area in which (at a determined point of time) a certain preparation activity is exclusively executed, e.g. a preparation of ointments. | The preparation area should be located in a less frequented zone or separated from the surroundings by protective walls reaching to the ceiling at least from three sides. | |
| Hygiene clothing | Hygiene clothing has to be designed in a way that it can ensure a product protection from contamination by human beings. | e.g. closed work coats made of cotton | |
| Hygiene concept | Totality of all hygiene measures | Hygiene programme | |
| Hygiene monitoring | Proof and documentation of successful hygiene measures | ||
| Hygiene status | Microbiological quality of pharmaceutical primary materials and preparations, packing materials, devices, personnel, clothing and premises | ||
| Non-sterile pharmaceutical preparation | Pharmaceutical preparations of microbiological quality according to category 2 or 3 | (8) | |
|
Pharmaceutical preparation |
Each drug intended for the use by human beings or at animals or similar products which are subject to sanitary legislation control. | (17) | The definition "pharmaceutical product" according to (17) is limited to the human field. |
| Purity, microbial | Quality and test characteristics of pharmaceutical primary materials and preparations. The microbial purity of different pharmaceutical preparations is defined by categories. | (8) | Microbiological quality; microbial quality; microbiological purity. |
I Master Hygiene Plan - Personnel Hygiene (A)
| What | Jewellery |
| When | before hand disinfection |
| How | Taking off, e.g. of rings, wrist watches, bracelets, long earrings |
| Who | All co-workers employed in preparation |
Annex
I Master Hygiene Plan - Personnel Hygiene (B)
| What | Long hair |
| When | before hand cleaning before begin of preparation |
| How |
bind or pin up hair |
| Who | All co-workers employed in preparation |
Annex
I Master Hygiene Plan - Personnel Hygiene (C)
| What | Hand disinfection, hygienical |
| When | as required when having contact with infectious material |
| How | 1. spread by rubbing according to instructions for use 2. let dry 3. afterwards hand cleaning |
| By means of | Permissible hand disinfection from dispenser |
| Who | All co-workers |
Annex
I Master Hygiene Plan - Personnel Hygiene (D)
| What | Cleaning of hands |
| When |
before begin of preparation |
| How | 1. careful cleaning with warm water and washing lotion 2. rinse thoroughly with warm water 3. dry hands |
| By means of | water from mixing faucet gentle washing lotion from dispenser paper towel from dispenser |
| Who | All co-workers |
Annex
I Master Hygiene Plan - Personnel Hygiene (E)
| What | Hand disinfection, surgical |
| When | after hand cleaning before working at the open product |
| How | 1. Cleaning of hands 2. Spread hand disinfectant by rubbing according to instructions for use 3. Let dry |
| By means of | Permissible hand disinfectant from dispenser |
| Who | Co-worker immediately before corresponding preparation works |
Annex
I Master Hygiene Plan - Personnel Hygiene (F)
| What | Skin care |
| When |
As required |
| How | Massage skin care product evenly into skin |
| By means of | Suitable emulsion base |
| Who | All co-workers |
Annex
I Master Hygiene Plan - Personnel Hygiene (G)
| What | Gloves, sterile |
| When | Contact with the open product Interventions into the preparation sequence (risk of microbial contamination) |
| How | Slip gloves over disinfected, dry hands Dispose gloves after work in waste bin, hand cleaning, if necessary hand disinfection |
| By means of | sterile one-way gloves |
| Who | Co-workers performing corresponding preparation works |
Annex
I Master Hygiene Plan - Personnel Hygiene (H)
| What | Hand protection |
| When |
Work with skin incompatible substances |
| How | after activity disposal of the one-way gloves
in litter bin (without external contact) multipurpose gloves are to be cleaned after each use, then dried and disinfected afterwards cleaning of hands, if necessary hand disinfection |
| By means of | one-way gloves, non-sterile multipurpose gloves |
| Who | All co-workers when performing corresponding preparation and cleaning work |
Annex
I Master Hygiene Plan - Personnel Hygiene (I)
| What | Hygiene clothing |
| When | all activities in the preparation area works at the open product |
| How | separate keeping of hygiene and street clothing exchange at least twice a week resp. according to soiling |
| By means of | Work coats, e.g. made of cotton |
| Who | All co-workers employed in preparation |
Annex
I Master Hygiene Plan - Personnel Hygiene (J)
| What | Head caps |
| When | Continuing activities at the open product preparation of Defekturen (preparation of commercial quantities of a product - as contrast to individual preparation) |
| How | hair must be completely covered |
| By means of | one-way head cap |
| Who | co-workers at open product |
Annex
I Master Hygiene Plan - Personnel Hygiene (K)
| What | Masks for mouth and nose |
| When | Co-workers suffering from respiratory tract diseases who perform
activities within the preparation area continuing activities at open product |
| How | nose and mouth have to be covered exchange of mask after two hours |
| By means of | one-way mouth mask |
| Who | co-workers at open product |
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (A)
| What | Devices in contact with product | How | By means of | Who |
| When | before start of preparation | disinfection | alcohol at appropriate concentration | pharmacy staff |
| When | if necessary in advance for short-term requirement | resp. sterilisation | dry heat steam sterilisation |
pharmaceutical staff |
| When | after preparation | cleaning | according to operation procedure | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (B)
| What | Devices not in contact with product | How | By means of | Who |
| When | before start of preparation | wipe with damp cloth | water with all-purpose detergent disinfectant |
pharmacy staff |
| When | before start of preparation | spray | disinfecting spray | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (C)
| What | Worktops | How | By means of | Who |
| When | daily repetition as required |
wipe with damp cloth | water with all-purpose detergent disinfectant |
pharmacy staff |
| When | daily repetition as required |
spray | disinfecting spray | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (D)
| What | Floor | How | By means of | Who |
| When | daily | clean with damp cloth | water with all-purpose detergent | pharmacy staff |
| When | twice a week | clean with damp cloth | disinfectant | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (E)
| What | Door handles | How | By means of | Who |
| When | daily | clean with damp cloth | water with all-purpose detergent; disinfectant |
pharmacy staff |
| When | daily | spray | disinfecting spray | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (F)
| What | Washbasins | How | By means of | Who |
| When | daily | clean with damp cloth | water with all-purpose detergent; disinfectant |
pharmacy staff |
| When | daily | spray | disinfecting spray | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (G)
| What | Windowsills | How | By means of | Who |
| When | weekly | clean with damp cloth | water with all-purpose detergent | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (H)
| What | Shelves, storage vessels | How | By means of | Who |
| When | weekly | clean with damp cloth | water with all-purpose detergent | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (I)
| What | Walls, doors | How | By means of | Who |
| When | monthly or as required | clean with damp cloth | water with all-purpose detergent | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (J)
| What | Windows | How | By means of | Who |
| When | monthly | clean with damp cloth | water with all-purpose detergent | pharmacy staff |
Annex
II Master Cleaning and Disinfection Plan - Devices, Preparation Premises and Equipment (K)
| What | Cupboards/drawers | How | By means of | Who |
| When | monthly | clean with damp cloth |
water with all-purpose detergent |
pharmacy staff |
Master Check List for Self Inspection and ensuring of the internal Hygiene Concept (A)
| Question | yes | no | Measure and person responsible to remedy matter |
| 1. Does a separate preparation area/preparation room exist? | |||
| 2. Is the preparation area closed at least from three sides to the height of the wall? | |||
| 3. Has been ensured that the preparation area is not used as opposed to the intended purpose? | |||
| 4. Has been ensured that no tea-drugs are processed at the preparation area? | |||
| 5. Is there an even and easily cleanable floor covering in the preparation area? | |||
| 6. Are the worktops even and easily cleanable? | |||
| 7. Has a written cleaning instruction for the precision scales and the fine scales been established? | |||
| 8. Is there an indication that the cleaning instructions according to question 7 have been observed? | |||
| 9. Are the necessary scales according to question 7 available at the preparation area? | |||
| 10. Has a cleaning instruction in writing been elaborated for the water bath? |
Annex
Master Check List for Self Inspection and ensuring of the internal Hygiene Concept (B)
| Question | Yes | No | Measure and person responsible to remedy matter |
| 11. Is there an indication that the cleaning instruction according to question 10 has been observed? | |||
| 12. Is the surface disinfectant intended for the worktops available for daily application? | |||
| 13. Has been ensured that the worktops are disinfected on every working day? | |||
| 14. Are the devices used for the preparation stored in a dry manner and are they protected from contamination? | |||
| 15. Has been ensured that the parts of devices which get in contact with the products are disinfected before preparation? | |||
| 16. Has been ensured that only aluminium tubes or dispenser cans are used for the packaging of hydrophilic creams and hydro-gels? | |||
| 17. Has been ensured that only certified primary containers are used for packaging if available? | |||
| 18. Are washing lotions and one-way towels available for hand cleaning? | |||
| 19. Is a dispenser with disinfectant for surgical and hygienical hand disinfection available? | |||
| 20. Is a surgical hand disinfection (disinfection after hand washing) carried out on a regular basis before performing preparational activities? |
Annex
Master Check List for Self Inspection and ensuring of the internal Hygiene Concept (C)
| Question | Yes | No | Measure and person responsible to remedy matter |
| 21. Are one-way gloves available? | |||
| 22. Has been ensured that co-workers can stay in the preparation area when working at the open product and has been prevented that they might be called away to the telephone or to serve customers? | |||
| 23. Has been ensured that hygiene clothing is only worn within the preparation area and not when serving customers? | |||
| 24. Has been ensured that no packs returned by customers get back into the preparation area? | |||
| 25. Has been ensured that the exterior surface of the containers with primary substances are cleaned or disinfected if required before bringing them into the preparation area? | |||
| 26. Has been ensured that reusable storage vessels are cleaned or disinfected if necessary before bringing them into the preparation area? | |||
| 27. Has a written working instruction for the derivation and storing of water for pharmaceutical purposes been established? | |||
| 28. Is there an indication that the deadlines for the storing of freshly produced or freshly opened water in containers are adhered to? | |||
| 29. Is an operational hygiene plan concerning personnel hygiene available in writing? | |||
| 30. Are the measures regarding personnel hygiene observed? |
Annex
Master Check List for Self Inspection and ensuring of the internal Hygiene Concept (D)
| Question | Yes | No | Measure and person responsible to remedy matter |
| 31. Is an operational cleaning and disinfection plan available in writing? | |||
| 32. Is there a documentation for regularly recurrent hygiene measures available? | |||
| 33. Are regularly recurrent hygiene measures in fact documented? | |||
| 34. Is current specialized literature regarding industrial hygiene available? | |||
| 35. Has been ensured that regular (internal) trainings in the field of industrial hygiene take place? | |||
| 36. Has been ensured that non-pharmaceutical staff is equally included into the trainings regarding the hygiene concept? |
Copyright © 2000 Institute for Dermopharmacy GmbH webmaster@gd-online.de